The last noted period when the ocean waters were that acidic was over 20 million years. The numbers tell the story:
- There has been a 40 percent increase in atmospheric carbon dioxide (CO2) levels since the start of the industrial revolution.
- There has been a 26 percent increase in ocean acidity from pre-industrial levels to today.
- If the current level of high CO2 emissions continues, the projected increase in ocean acidity by 2100 – compared with pre-industrial levels – is predicted to hit approximately 170 percent.
- The current rate of acidification is over 10 times faster than at any time in the last 55 million years.
- The ocean absorbs 24 million tons of CO2 every day.
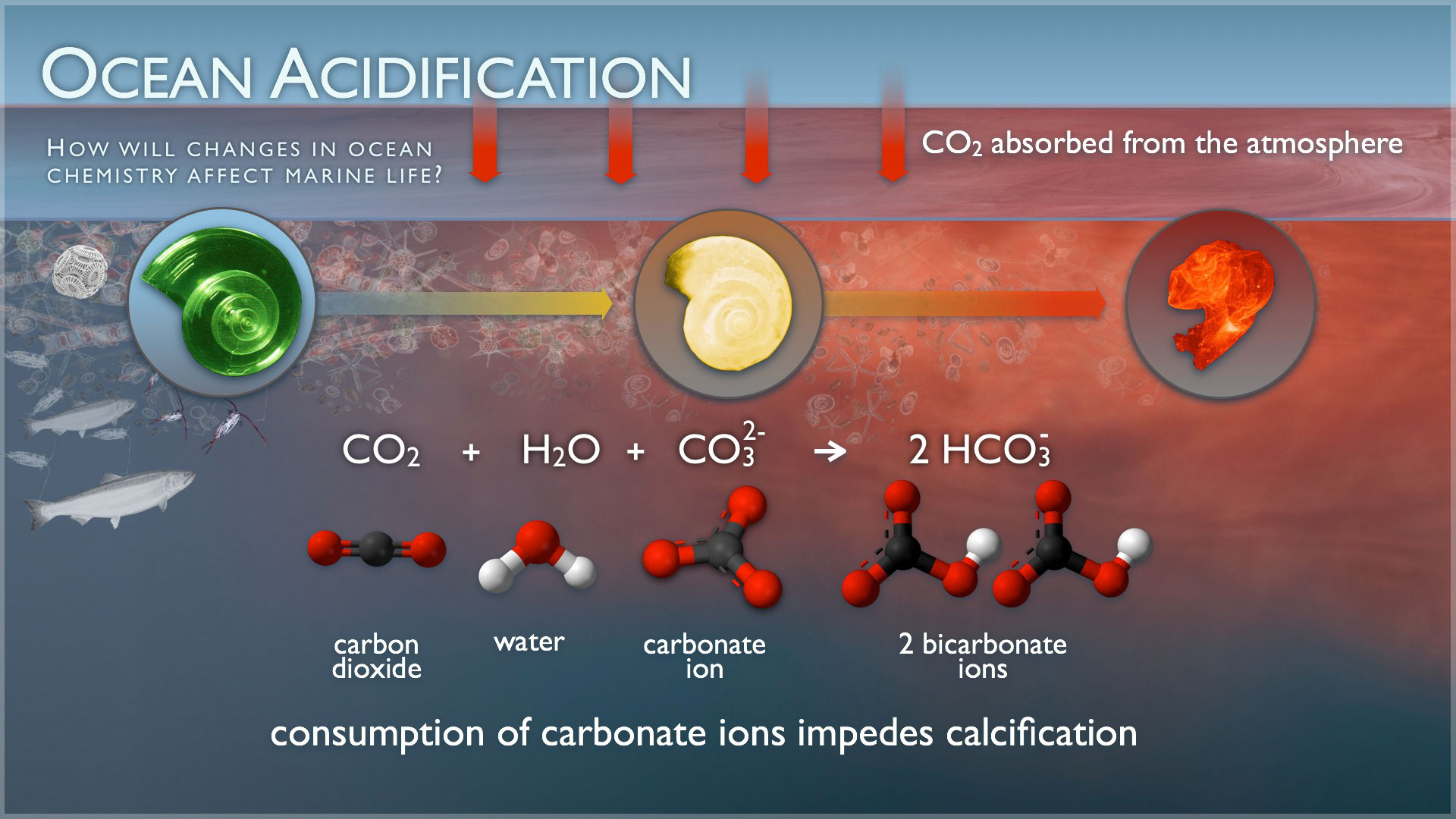
Ocean acidification will impact ocean species on a number of levels. While photosynthetic algae and sea grasses may benefit from higher CO2 conditions in the ocean, a more acidic environment has a very different effect on calcifying species. Oysters, clams, sea urchins, shallow water corals, deep sea corals, and calcareous plankton – as well as any other shelled organism – are at risk, thus putting the entire food web at risk. We, as humans, are a part of that web; over a billion people worldwide rely on food from the ocean.
The effects on sea life vary by species. Some specific examples are;
The Pteropod, or “sea butterfly” – this is a tiny sea creature; pteropods are eaten by organisms ranging in size from tiny krill to whales and are a major food source for North Pacific juvenile salmon. The pteropod’s shell when placed in sea water with pH and carbonate levels projected for the year 2100 dissolves after 45 days.
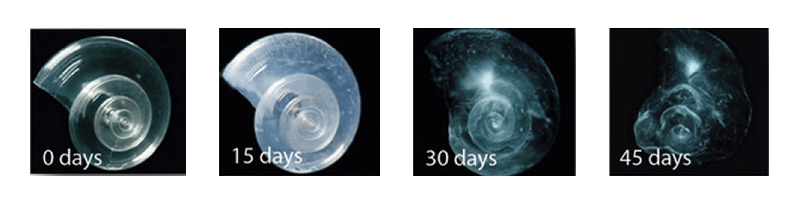
Reference: http://ngm.nationalgeographic.com/2007/11/marine-miniatures/acid-threat-text
Shellfish – Developing oysters in both aquaculture facilities and natural ecosystems on the West Coast have seen a sharp decline due to larval oyster failures. These failures correlated with naturally occurring upwelling events that changes water quality in near shore environments. Researchers are observing that anthropogenic CO2 is contributing to seasonal under saturation. Low pH is a factor in the current oyster reproductive failure. Acidification is a potential factor in the current crisis to this $100 million a year industry.
Coral – Increasing ocean acidification has been shown to significantly reduce the ability of reef-building corals to produce their skeletons. As a recent example, coral biologists report that ocean acidification could compromise the successful fertilization, larval settlement and survival of Elkhorn coral, an endangered species. Ocean acidification could severely impact the ability of coral reefs to recover; by the end of this century, coral reefs may erode faster than they can be rebuilt.
Ocean acidification is a global problem. While ocean acidification has been highlighted during the last decade, there is much that still needs to be done. Sustained efforts to monitor ocean acidification worldwide have begun. However, it is impossible to predict to what degree ocean acidification will impact the marine food chain and the overall structure of marine ecosystems in the future. One thing the science community does agree on is that ocean acidification is accelerating.